The Grignard reaction is a general term for various chemical reactions involving Grignard reagents.
In 1900 the French chemist Francois Auguste Victor Grignard discovered that organohalides react with magnesium in ether solvents to form organomagnesium compounds (eq. 1), these compounds in turn reacting with ketone and aldehydes to produce alcohols. These organomagnesium compounds were named Grignard reagents, after their discoverer.

In addition to the chemical reactions of Grignard reagents with ketone and aldehydes, reactions with esters, amides, acid chlorides, carbon dioxide, nitrile, alkyl halides, metallic halides and other substances are widely used today. New Grignard reactions are still undergoing intense study and search at present.

Despite their high reactivity with various compounds, Grignard reagents seldom catch fire and are easy to handle, as in the cases of organolithium, organosodium and other organometallic reagents. However the synthesis reaction of Grignard reagents is accompanied by vigorous heat generation, since the reaction requires an induction period. The reaction heat must therefore be controlled appropriately when the reagents are mass-produced on an industrial scale. Because of the highly reactive and easy-to-handle properties of Grignard reagents, they are widely used to produce various compounds at both industry and laboratory levels, despite the difficulty of controlling reaction heat during the synthesis of these compounds.
As equation 1 shows, Grignard reagents are usually synthesized by reacting an organohalide with metallic magnesium, mainly in an ether or tetrahydrofuran (THF) solvent in an inert gas atmosphere (eq. 1).

●General properties of Grignard reagents
- ・ Although Grignard reagents are originally colorless solids, the synthesized reaction liquids generally assume a color between light and dark gray.
- ・ Since Grignard reagents readily react with atmospheric oxygen or moisture, the surrounding air must be replaced with an inert gas (nitrogen or argon) when such agents are synthesized or stored.
- ・ In equation 1 given above, the reactivity of organohalides with magnesium is in the descending order RI, RBr and RCl.
- ・ Grignard reagents dissolve readily in aliphatic ether, alicyclic ether, dioxane or other solvent, thereby producing their adducts.
- ・ Grignard reagents are highly reactive with water, alcohols, amines, acetylene and other compounds containing active hydrogen, as well as with halogen and carbonyl compounds.
- ・ A small amount of iodine, methyl iodide or 1, 2-dibromoethane is used, when needed, as a reaction initiation agent.
1. Nucleophilic addition reactions with Grignard reagents
Grignard reagents react with various electrophilic compounds.
One of the most typical electrophilic reactions is an addition reaction with an aldehyde, ketone or other carbon-oxygen double bond that synthesizes a secondary or tertiary alcohol (eqs. 2 and 3).
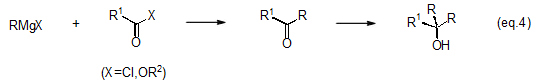
Reaction of a chloride, carboxylic acid, acid anhydride or ester with excess Grignard reagent creates two types of alkylated tertiary alcohols (eq. 4).
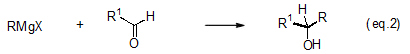
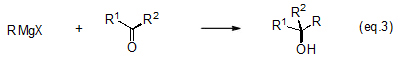
Because nitrile is less reactive than these carbonyl compounds, it has long been used to synthesize asymmetrical ketone (eq. 5).
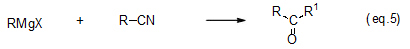
The use of formaldehyde as an aldehyde for the reaction produces a primary alcohol containing one more carbon atom, while the use of ethylene oxide yields a primary alcohol containing two more carbon atoms (eq. 6).

A reaction with carbon dioxide yields carboxylic acid having one more carbon atom (eq. 7).
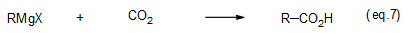
2. Nucleophilic displacement reaction with Grignard reagents
Grignard reagents are seldom used for synthesis reactions, because they are less reactive with halides and other substances than are organolithium reagents. However when a transition-metal compound is added, the compound acts as a catalyst for efficient production of coupling products (eq. 8).
In the 1970s, cross-coupling reactions between Grignard reagents and halides, with a transition metal as catalyst, were studied extensively. Study results revealed that Fe, Co, Ni, Pd, Cu, Ag and many other transition metals exhibit catalytic actions. Ketone synthesis by means of a transition metal catalyst is widely known as a typical example of study results (eqs. 9 thru 10).
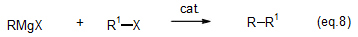


Since alkyne hydrogen is highly acidic, it reacts with a Grignard reagent to form an alkynyl magnesium halide (eq. 11).
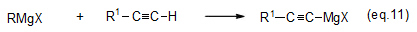
Reacting a Grignard reagent with a metal halide enables the synthesis of an alkyl metal compound. This reaction is used to industrially produce organometallic compounds (eq. 12).
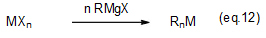
Production of Grignard reagents requires various industrial techniques for raw material and solvent dehydration/drying, reaction heat reduction etc. Grignard reagents produced by employing these techniques are used to produce organometallic compounds (organotin compounds, organosilicon compounds, organoboron compounds etc.), as well as primary materials and intermediates of pharmaceuticals and agrochemicals.
Organometallic compounds
Grignard reagents are widely used to produce organometallic compounds on an industrial scale. The following are typical examples of metals, semimetals and other metallic species that are produced industrially, and their applications.
(1) Organotin compounds
Various organotin compounds can be produced by reacting Grignard reagents with tin tetrachloride.
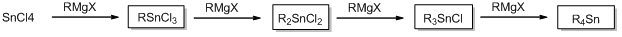
Organotin compounds are used as stabilizer for vinyl chloride resins, catalyst for hardening urethane, catalyst for hardening silicon resin, and other industrial purposes.
(2) Organosilicon compounds
Combinations of Grignard reagents and suitable types of raw silicon compounds enable the production of various kinds of symmetric and asymmetric di-, tri- and tetra-organosilicon compounds (eqs. 13 thru 15).
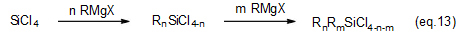
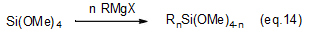

These compounds are used as protective groups in organic synthesis, metallocene catalyst materials for olefin polymerization and intermediates in pharmaceutical synthesis.
(3) Organophosphorous compounds
The phosphine compound is a typical example of industrially useful organophosphorous compounds produced using Grignard reagents. Phosphine compounds, which can be made from Grignard reagents and halide phosphates (eq. 16), are used as Wittg reagents for vitamin synthesis, the additives for various synthetic resins, and for other applications.
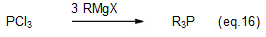
Phosphonium salt, R3PR’X, which can be produced by reacting the above R3P with an alkyl halide, is useful as a phase-transfer catalyst.
(4) Organoboron compounds
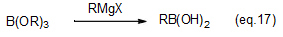
Phenylboronic acids, which are used for Suzuki-Miyaura cross-coupling reactions, are synthesized from Grignard reagents and boric-acid esters (eq. 17).